The Grading of an Energy Source
Revised 24 May 2019
Different forms of energy differ in their ability to do useful work. A calorie of dispersed heat cannot do any work. Sunlight must first be concentrated to be able to do useful work. However, different kinds of energy are not equally convertible into useful work. It takes energy to concentrate energy. Some energy must be degraded in order to concentrate what is left.
Figure 13 shows the scale of quality of energy and some of the conversion factors for going from one form of energy to another. These factors include the energy cost of any machinery that the conversion process might require.
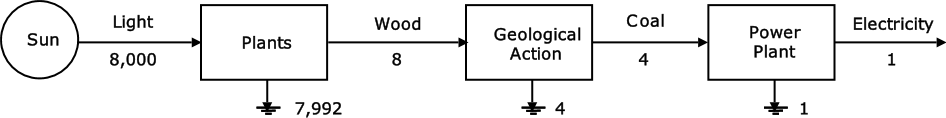
Figure 13: Scale of Energy Quality (Odum, 1976, p32)
The degree of convertibility of energy - stored work - into applied work is often called availability. Energy in forms having high availability is called high-grade energy. Low-grade energy is the energy which only a small fraction can be converted to applied work. An example of high-grade energy is the energy stored in fossil fuels and electricity. Sunlight is an example of low-grade energy.
Thermal energy is a special case. The greater the difference between the heat source and its environment, the greater is the availability. The hot core in a nuclear reactor is energy of high availability, while that of a domestic radiator is of low availability or low-grade energy.
Figure 14 shows that human activity is involved with the conversion of low-grade energy to high-grade energy. This high-grade energy has greater availability to do useful work.
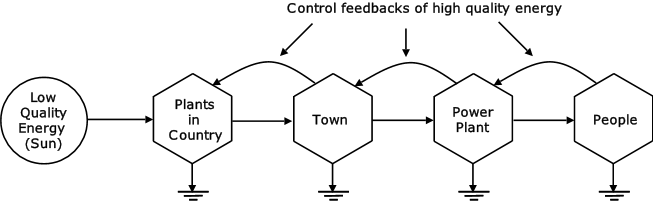
Figure 14: Energy Conversion (Odum, 1976, p77)
High-grade energy can be used in conjunction with a larger flow of low-grade energy to produce high-grade energy. Figure 15 shows a flow of 10 units of high-grade energy being used to transform a flow of 100 units of low-grade energy into a flow of 20 units of high-grade energy. During the transformation process a flow of 90 units of heat energy is generated.
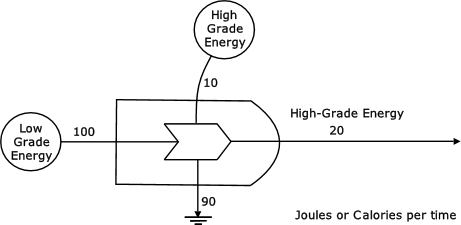
Figure 15: High grade energy acting as an amplifier of low-grade energy (Odum, 1976, p78)
Figure 16 shows a flow of 10 units of high-grade energy being transformed into a flow of 2 units of medium-grade energy. During the transformation process a flow of 8 unit of energy is generated. High-grade energy is wasted if it used for purposes which can be provided by using low-grade energy. An example is using an electric bar heater to heat a room in a home when passive solar house design can achieve the same result.
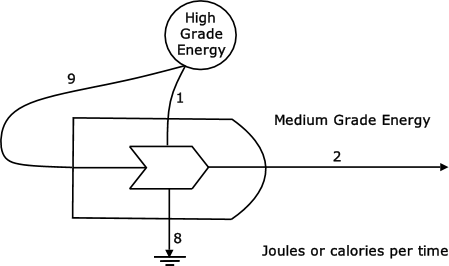
Figure 16: Wasteful use of high-quality energy to produce medium-quality energy (Odum, 1976, p78)
The grading of an energy source can be classified in terms of the energy level of the source ─ a measure of its energy intensity in terms of energy per unit mass ─ and its energy grade that is a measure of energy quality. A high energy level system has the characteristics of high temperature, pressure, or enthalpy (heat of combustion).
The energy grade of a source can be separated into either work forms or heat forms of energy. Work forms of energy include mechanical work, electrical energy, waterpower, wind power, and the kinetic energy of a jet stream. Work can be completely transformed into heat, but the reverse transformation of heat cannot completely transform heat into work due to the Second Law of Thermodynamics. Work forms of energy are therefore given a higher classification than heat forms. This distinction becomes less significant with high temperature sources of energy.
Heat forms of energy ─ heat from fusion/fission, heat from combustion, and heat from friction ─ are graded in descending order according to the temperature of the source. The energy grade of a source serves to qualify the energy level. When two energy sources have the same level but different grades, the ordering of the lower grade source can be adjusted downwards to reflect a lower availability.