Minerals Needed for Batteries versus Reserves
Revised 2 December 2023
We need to transition from fossil fuels to renewable energy and infrastructure, and there is a common assumption by many that renewables can provide the same scale of net energy that fossil fuels have provided so far. Simon Michaux (2019) has challenged that assumption with a study of the minerals and metals required by renewables.
In Figure 1, the global reserves of metals as of 2018 needed by batteries to phase out global fossil fuels are coloured blue and the metals needed are coloured yellow. Copper is currently not an immediate problem, but nickel, cobalt, lithium, and graphite present serious problems. Global reserves of nickel, cobalt, and lithium for batteries are simply not large enough to supply enough metals to scale up renewables to fully replace the global net energy currently provided by fossil fuels.
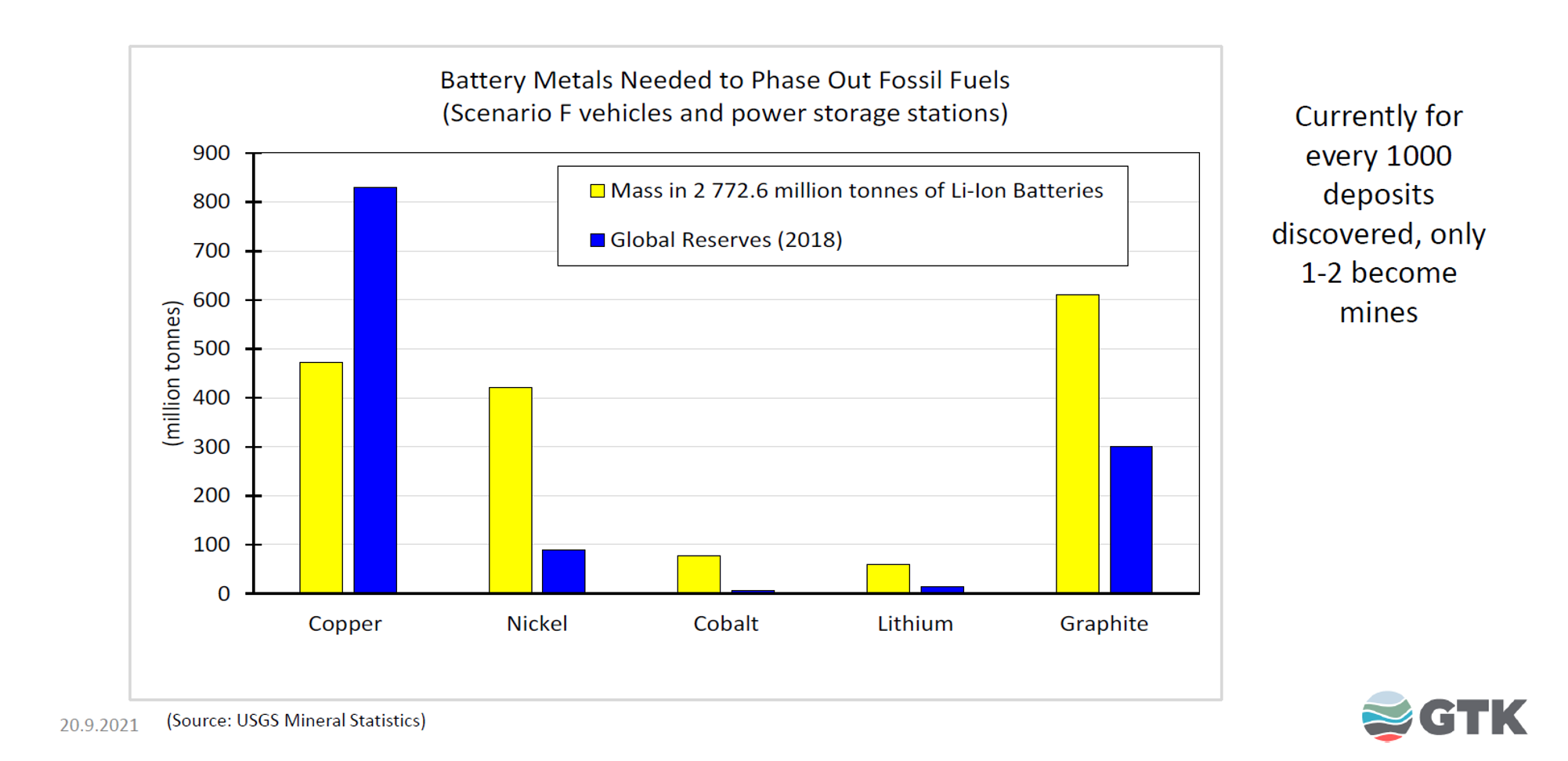
Figure 1: Minerals Needed for Batteries versus Reserves (Michaux 2019)
Michaux (2019) concluded that alternative minerals will be needed for batteries. These include zinc, sodium-sulphur, and hydrogen fuel cells.
In Figure 2, abundant elements are coloured green and those elements which pose a serious supply threat over the next 100 years are coloured red. The reserves of zinc present a serious supply threat over the next 100 years while sodium and sulphur are abundant.
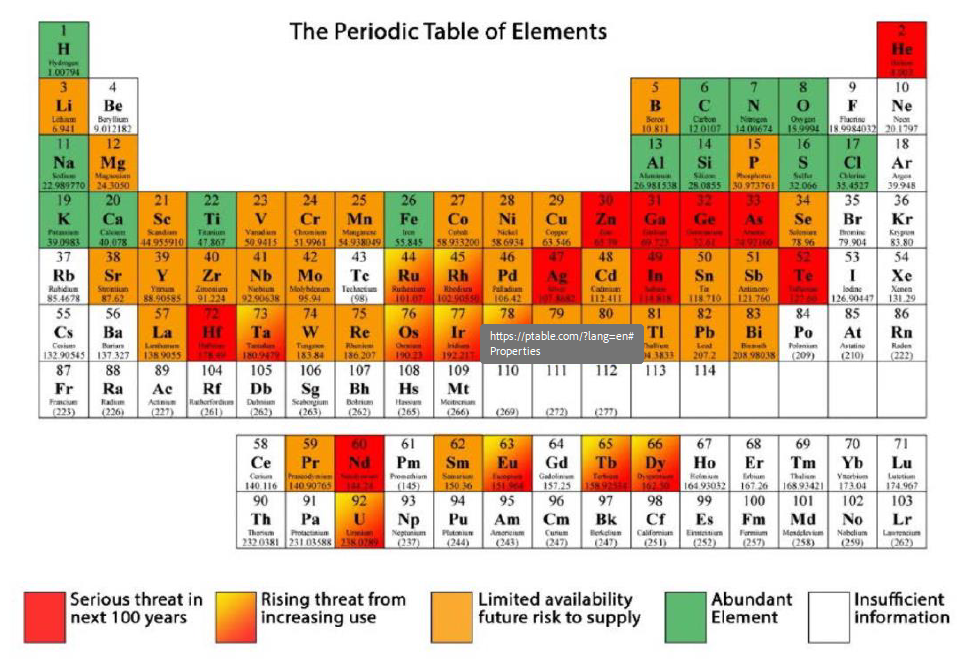
Figure 2: Primary Mining Sources with Supply Risks (Michaux 2019)
There are risks involved with handling sodium and sulphurs due to the volatile nature of both reactants. Liquid sodium can become explosive when coming into contact with water in the atmosphere. Sodium-sulphur battery factories and installations that use them have been the site of several fires. Another drawback to sodium-sulphur batteries is the high operating temperature of 300 °C, which is needed to liquefy the sodium. These high temperatures can damage the ceramic membrane separating the anode and cathode components of the battery and exacerbate the volatility of the reactants in the battery. (Moseley & Garche 2014).
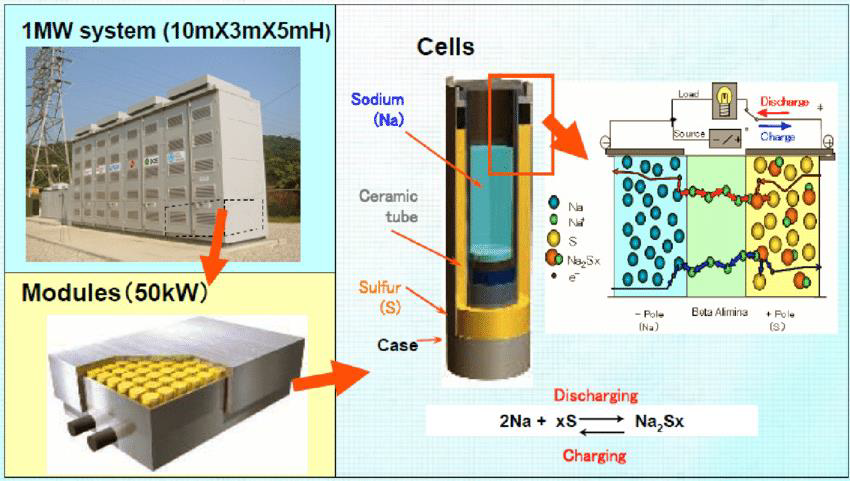
Figure 3: Sodium-Sulphur Batteries
Hydrogen is highly flammable and can react explosively if not handled correctly. Hydrogen is an energy carrier and not a direct source of energy because the production of hydrogen is 65-80% efficient. In other words, it takes more energy to produce hydrogen than the energy contained in the hydrogen that has been produced. There are further energy losses in compression of hydrogen and loss of energy in a fuel cell when supplying energy to an electric motor to undertake the mechanical work of motion. Fuels cells are about 65% efficient in supplying the energy of hydrogen to an electric motor. Electric motors also have a loss in energy due to friction when used to undertake mechanical work of motion. (Murphy 2021).
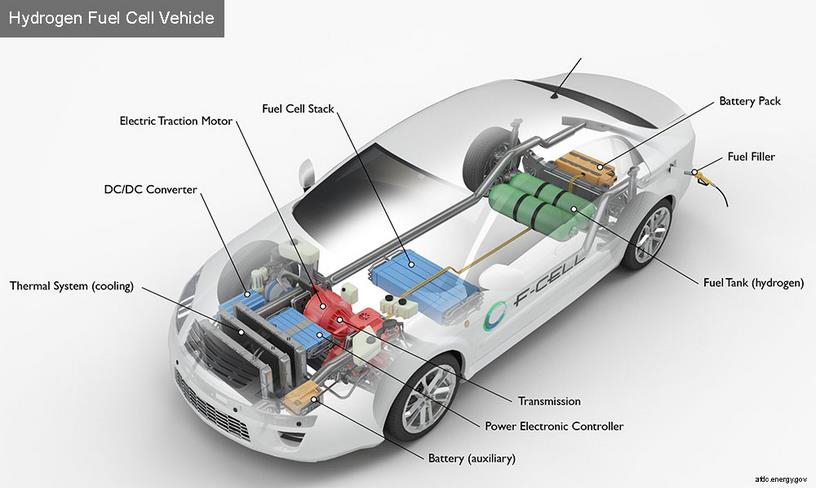
Figure 4: Hydrogen Fuel Cell Vehicle