First & Second Law Effiiciencies
Revised 24 May 2019
We are concerned with how efficiently energy is used in transport, industry, agriculture and many other processes. By using energy in efficient ways, not only do we use less of our non-renewable resources with subsequent reductions in greenhouse gas emissions, but also less energy ends up in the environment as low-grade heat.
The First Law Efficiency is the ratio of the amount of energy delivered to perform a task to the amount of energy that must be applied to achieve this task. This first law approach is concerned only with the efficiency of one particular method of performing the task, and disregards alternative methods which may perform the same task with less energy consumption. The second law efficiency, on the other hand, is the ratio of the minimum amount of available work needed to perform a task to the actual amount of available work used to perform this task. The second law efficiency approach focuses on the task at hand and gives a measure of how much improvement in performance is theoretically attainable.
The first law efficiency is the ratio of the energy delivered by the process in the form and location necessary to achieve that task to the amount of energy supplied to the process. The first law efficiency can be used as a measure of energy conservation in carrying out a task but, in doing so, the quality of energy conserved is not taken into account. There is no differentiation between energy losses caused by imperfections in the energy conversion process. Energy losses due to the Second Law of Thermodynamics cannot be avoided even by perfect technology. These factors are included in the second law efficiency.
The Second Law Efficiency is the ratio of the minimum amount of available energy required to carry out a task to the actual amount of available energy used. The second law efficiency is a measure of how much the performance of a task falls short of what is theoretically possible, and can be used as a measure of the conservation of free, or available energy (exergy) in carrying out a task.
An examination of the task of heating a house provides an illustration of the difference between first and second laws efficiencies. In an example provided by Ehrlich et al. (1977), a standard furnace is able to deliver 1 unit of energy for heating a house for every 1.5 units of energy extracted from its fuel. The first law efficiency is
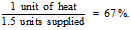
By using the most efficient Carnot heat pump where the Coefficient of Performance (COP) is solely dependent on the temperature difference inside and outside the house, the minimum amount of available work required to deliver 1 unit of heat is 0.07 units. The available work in a chemical fuel is approximately equal to its heat of combustion or enthalpy. The available work used by the furnace remains at 1.5 units. The second law efficiency is
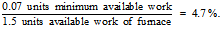
The second law efficiency is based on comparing actual processes with idealistic processes that do not necessarily include a realistic time frame. There is a trade-off between efficiency and power. An infinitesimally slow reversible process may be carried out with maximum efficiency, but with a penalty of a power output approaching zero. A very rapid process, on the other hand, approaches a maximum power input but at zero efficiency and zero power output. Life forms and the activities of humankind require energy processes to be carried out at an intermediate range of rates that fall well short of the maximum second law efficiency. Odum & Pinkerton (1955) proposed that natural systems tend to operate at an efficiency that produces a maximum power output, but Peet & Baines (1986) caution that although the Maximum Power Principle represents deduction from a wide range of empirical observations, its universality has yet to be proved or generally accepted.
Moore (1981) recommends the following guidelines to ensure that the conservation of free or available energy (exergy) is maximised when energy is supplied in a converted form in order to carry out a task.
- Firstly, there should be a minimum number of energy conversion steps. Each unnecessary energy conversion step involves an unnecessary loss in free energy because there is a severe penalty in transforming the energy of heat into mechanical work.
- Secondly, heat should be converted into work at the highest possible temperature and should be undertaken only once.
- Thirdly, the direction of any series of energy conversion processes should proceed from those with maximum conversion efficiencies to those with a lower efficiency of conversion.
- Fourthly, energy should ideally be stored in work reservoirs such as compressed air, mechanical springs, and pressurised liquids because such devices provide the potential for minimal energy storage loss.